Two of the five strategic objectives in the Global action plan on antimicrobial resistance explicitly link to estimating the cost burden of antibiotic resistance and/or quantifying the economic effects of a given policy.
The action plan explicitly states the need for models which assess the costs and benefits of policies within the national action plan across human health, animal health, food, plant and environmental sectors. Cost-benefit and cost-effectiveness models (built using data from the burden measures and disease epidemiological measures covered in MEASURE) can provide policy makers with evidence on the financial incentive to invest further in tackling antibiotic resistance.
Human health case study: Cost-effectiveness modelling of antimicrobial stewardship programs in a Brazilian hospital
Description: Estimation of the cost-effectiveness of two antimicrobial stewardship programs using hospital data. A model similar to the one drawn out below was used, where arrows depict the potential movement of patients. The conventional strategy was compared with a bundled policy intervention with prospective auditing, feedback, education, and microbiological data discussion with laboratory staff. The model included costs of staff, defined daily doses/patient, length of stay, and laboratory and imaging resources used to diagnose infections.

Place: Brazil
Setting: University hospital, 550 beds.
Finding: The “bundled” policy intervention was more cost-effective, with a cost per averted death in 30 days of US $19,290 (2013 USD). Although this study was based in one hospital, sensitivity analysis suggested that this result was robust. However, only a short time frame was used in this case study (30 days). Future evaluations should try and include the potential impact of the policy on resistance in the longer term.
National models utilizing similar methods can be constructed with parameters measured in previous national, regional or local studies. These models can compare the population-level effect of treatments under different policy scenarios and estimate the difference in cost-effectiveness (incremental cost-effectiveness ratio, ICER). If some data are unavailable, the next best-estimate can be used. Uncertainty can be quantified through sensitivity analyses.
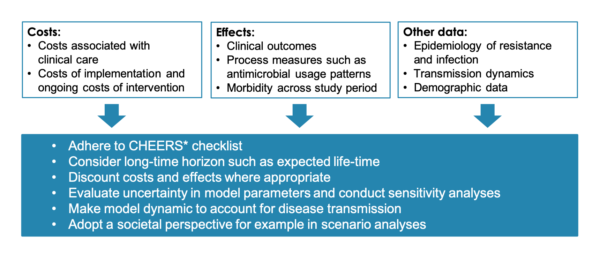
When estimating the cost-effectiveness of antimicrobial stewardship programs in humans, a literature review recommended:
Food animals case study: Antibiotic policy scenarios modeled in a cohort of dairy cows
Description: A dairy farm simulation was used to model net cost differences of different antibiotic usage policies. A baseline simulation was created of a typical farm operation. This was compared to simulations of four policy strategies from which two included, (i) a complete ban on antibiotic use in dairy cows and (ii) a policy of substitution of therapies. A stochastic simulation model was used to estimate cost differences over a two-year period, but did not include the potential longer-term benefits of reduced antibiotic usage.
Place: “Generic western” country
Setting: Simulated dairy farm, 1000 cows
Findings: From the farm’s perspective, the model predicted that there would be an average cost increase of $150 and $61 per cow per year for policy (i) and (ii) compared to the current baseline strategy. The cost difference increased with disease prevalence in all modeled scenarios. Additionally, cow replacement prices, cow slaughter price, and the milk price were shown to significantly impact the estimated cost difference. Disease transmission between animals, and potential adjustments by farmers, were not considered in this model.
Future models should attempt to model monetary costs and benefits over a longer time horizon and include adjustments by farmers. Although the model assumed an average level of disease caused by the nine most frequent bacterial dairy diseases found in western countries, a similar modelling process could potentially be used in different settings.
Selected Resources
| Resource | Description |
| Health Systems Governance and Financing | Information portal on health financing. The WHO CHOICE (CHOosing Interventions that are Cost- Effective) project for example offers guidance on what to consider when conducting cost-effectiveness analyses. See “New cost-effectiveness updates from WHO-CHOICE” for recent publications and advice (incl. Methods for the Economic Evaluation of Health Care Interventions for Priority Setting in the Health System: An Update From WHO CHOICE). See also the The OneHealth Tool, a software tool designed to inform national strategic health planning in LMICs. It links objectives and targets of disease control and prevention programs to the required investments in health systems. |
| Quantifying environmental health impacts: Considerations in evaluating the cost effectiveness of environmental health interventions | This WHO report offers guidance on methods for evaluating environmental health interventions. |
| Investment Learning Platform (ILP): Financial and Economic Analysis | This FAO-based website provides resources on how to conduct economic evaluations in the food and agricultural sector. For example, one resource is entitled “Economic analysis of agricultural projects” |
| Introduction to health economics for the medical practitioner | This article gives an overview of what health economics is and what different types of analyses can be performed. |
| Network for Evaluation of One Health | This website acts as a repository for all of the work, conferences and publications associated with the Network for Evaluation of One Health. This is an EU-based organization, although the methods used and discussions can be applied internationally. |
| The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) | Comprehensive checklist to support reporting of economic evaluations of health interventions. Checklist for reporting. |