2021-10-28
Effective antibiotics are a cornerstone of basic and specialized medicine. The emergence of resistance in bacteria to antibiotics is slowly dismantling our ability to treat infections, alleviate human suffering, and save lives. The COVID-19 pandemic is a clear reminder of the deadly consequences the world faces, when we do not have the right treatments or vaccines available when needed.

It is widely accepted that in order to keep pace with accelerating antibiotic resistance – three actions are needed:
- Existing antibiotics must be kept effective including through infection prevention strategies and by optimizing their use through stewardship.
- Access to existing effective antibiotics must be expanded to everyone in need.
- The antibiotic pipeline must be filled and continually replenished to allow new effective types of antibiotics to be developed.
The innovation void
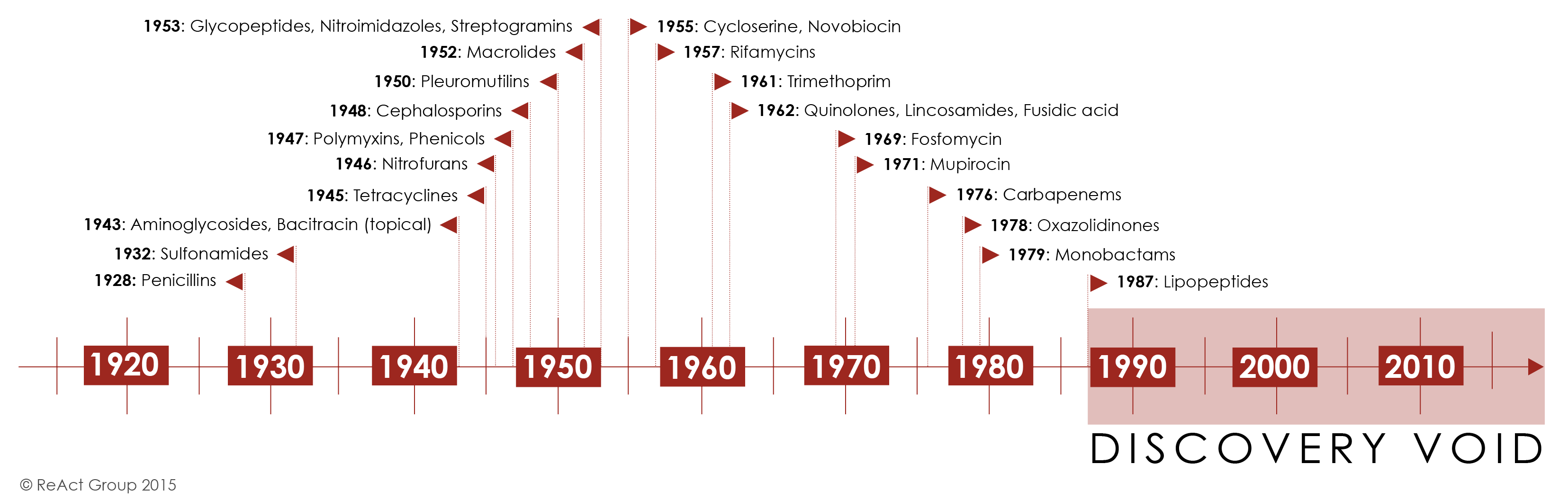
It has been 34 years since the last class of antibiotics was discovered. All antibiotics discovered since then are modifications of existing classes which means that resistance to them may develop faster. With resistance on the rise, the medical need for new classes of drugs is therefore clear. So why isn’t it happening?
Over three decades of standstill in antibiotic innovation can partly be explained by a difficult underlying science. Promising compounds are harder to find today as the low-hanging fruits in antibiotic discovery were picked between the 1960s and the 1980s. Historically, it is estimated that traditional antibacterial drugs have a ten-fold lower yield in the discovery stage of identifying promising new compounds when compared to all other drug classes. Many of the scientific challenges leading to these higher failure rates, such as penetration issues, efflux, and managing toxicity, remain unresolved and will still affect any traditional antibiotic compounds being researched and developed today (1).
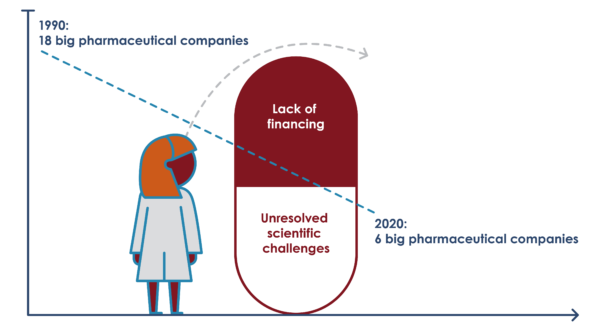
Withdrawal of the big pharmaceutical industry
The difficulty in discovering and developing new antibiotics has been further complicated by an overall withdrawal from the field by the pharmaceutical industry, leading to declining investments and loss of expertise and human resources in the antibacterial field. Over the last two decades, the number of multinational companies with active anti-infective programs fell from 18 to just six in 2020 (as of March 2021: Merck, Shionogi, GSK, Pfizer, Otzuka, and Johnson & Johnson).
The scientists’ challenge
In 2011, Pfizer shut down its primary antibiotic research center and relocated the research facility to Shanghai which at the time was regarded as a “crushing blow” to the field of anti-infectives research. Since then, a number of big companies have followed suit. Between 2016 and 2018, four big companies – AstraZeneca, Sanofi, Novartis (including its subsidiary, the Medicines Company), and Allergan – all exited from antibiotic R&D. In 2014 Merck bought up longstanding anti-infectives company Cubist (which was then widely touted as a sign of big pharma re-entry). However, the optimism was short-lived. Just 3 months later Merck fired 120 researchers and Cubist’s early-stage discovery research unit was closed down.
The extensive withdrawal of the research-intensive, multinational pharmaceutical industry was a deleterious outcome of their dependence on the blockbuster business model (with annual profit expectations at the billion dollar level). With such extreme profit expectations in the general pharmaceutical market, a field like antibiotic development (where profits in most cases are lower – Daptomycin being an exception) has few chances of being prioritized.
New actors have stepped up
Numerous smaller biotech companies have since filled the gap in the early stages of clinical development. However, these actors often focus on developing only a few compounds, often rely on public financial support and generally have limited experience in bringing new drugs all the way to market.
In fact, The Pew Charitable Trusts estimates that 70% of the small companies involved in development of new antibiotics have no previous experience at all with bringing a product to the market. Those that have succeeded, such as Achaogen and Melinta Pharmaceuticals, have failed to earn sufficient near-term revenues in the US market to sustain their businesses. Over a twelve-month period in 2019 and 2020, both companies filed for bankruptcy.
Achaogen’s bankruptcy has since been used as an example of the dysfunctional market for new antibiotics (ie. that even a drug that has is listed on the WHO’s essential medicine’s list is unable to generate a profit), and that governments by extension should provide large incentives to developers to fix the profitability problem. However, the reasons for the failure of Archaogan’s drug Plazomycin is far more complex (see detailed description in grey box further down). The bankruptcy shows that markets in high-income countries actually works in the sense that drugs with limited clinical benefits over existing, still effective treatments (which was the case for Plazomicin for UTIs in the U.S.) provide smaller financial returns.
It also serves as an important reminder that it is not enough that there is a big global medical need for the drug – many patients do not equal a large market, unless they are based in lucrative high-priced markets such as the EU and US. There is no guarantee in the current system that subsidies to developers would yield additional access beyond these regions.
What way forward then?
The now more than 30 years innovation gap is convincing evidence that the world cannot continue to leave it to ”the market” to develop antibiotics. It should therefore be clear to policy makers, that more of the same will not be the answer.
Many new antibiotics will need to be sparingly used as last line treatments to preserve their effect for as long as possible, which means that the commercial market will be limited. However, a novel antibiotic may also directly become a 1st line treatment due to disease patterns and resistance levels to existing drugs (as will likely be the case for a new gonorrhea treatment). In such cases the commercial market will likely be big from day one.
A missions-driven end-to-end approach
In either case, the market-based model which relies on large sales volumes and high prices of a new drug will not be efficient nor appropriate to ensure global sustainable access.
Novel approaches are required at all stages from early research and development to patient access. Global and national discussions need to move away from focusing on “market fixing” which often limits discussions to only focusing on how to re-enlist the big multinational pharmaceutical companies within the constraints of their traditional business model.
Instead, clear public leadership is to define a missions-driven end-to-end vision for development of and sustainable access to effective new antibiotics. New alternative and transformative models of financing, collaboration, IP management, production and distribution needs to be explored.
The concept of delinkage is central
A crucial part of such models is “delinkage”, which has been acknowledged for its potential to ensure affordable access without excess use of antibiotics (3).
”We acknowledge the importance of delinking the cost of investment in research and development on antimicrobial resistance from the price and volume of sales so as to facilitate equitable and affordable access to new medicines, diagnostic tools, vaccines and other results to be gained through research and development.”
– UN Political Declaration on AMR, 2016
Delinkage was originally developed as a way to overcome the lack of R&D for neglected tropical diseases, which primarily affect people in low-income countries and therefore not regarded as commercially relevant for drug companies. In its original form, delinkage only dealt with severing the link between R&D costs and final price of the drug, to secure affordability. However, antibiotics are crucially different in that the cost of R&D needs to be separated from both high prices and sales volumes, which ReAct has played a critical role in highlighting (2).
This makes the model for antibiotics different from both the conventional market-based pharmaceutical innovation model, which relies on maximizing sales of high-priced drugs in key high-income markets, and the generic pharmaceutical business model, which is based on low price/high volume sales in all countries.
A delinked pharmaceutical Research and Development model
The case of Plazomicin

The commercial pull of the U.S. market, which represents roughly half of the global pharmaceutical market, means that companies often file for registration first in the United States. However, the bankruptcy of the company Achaogen in 2019, which only registered its flagship product, the antibiotic Plazomicin, in the United States, illustrates how such a strategy can fail.
No alternative treatment options
Achaogen received a “priority review designation” from the U.S. Food and Drug Administration (FDA) for the antibiotic Plazomicin, which is a modified compound from an already existing class of antibiotics – aminoglycosides. This meant that the drug was put at the front of the line for review by the FDA and was subsequently approved in 2018 for treating urinary tract infections (UTIs) caused by drug-resistant bacteria in patients with no alternative treatment options. The FDA, however, did not approve a second indication – treating resistant bloodstream infections caused by Carbapenem-resistant Enterobacterales (CRE) – for which Achaogen had also sought market approval, as not enough patients had been enrolled in a trial for that indication.
Plazomicin included in the WHO’s Essential Medicines List
Plazomicin was included relatively quickly in the WHO’s Essential Medicines List within the so-called “Reserve” category of last line antibiotics to treat certain multidrug-resistant infections – a testament to the need for the drug globally. However, Achaogen only registered and made the drug available commercially in the United States. After regulatory approval, Plazomicin did not sell as much in the U.S. as expected (likely due to the limited approval for use in treating resistant UTIs, where other treatment options were already available). Analysts had projected peak sales worth around 500 million USD for Plazomicin. However, six months after it was launched, sales revenues were less than one million USD. This was insufficient to sustain Achaogen’s operations, and the company filed for bankruptcy in 2019, only one year after the FDA’s approval of Plazomicin.
Plazomicin was considered commercially and financially unviable
During Achaogen’s bankruptcy, Plazomicin was acquired by the Indian pharmaceutical company, Cipla, which applied for market approval at the European Medicines Agency (EMA). Cipla subsequently withdrew the application, as the product was considered commercially and financially unviable, given the costs of generating the necessary approval and post-approval data required for an EMA market authorization.
Drug with limited clinical benefits over existing treatments – prove smaller financial returns
Achaogen’s bankruptcy and Cipla’s decision to withdraw its application at the EMA serves as an important reminder that the market actually works, in the sense that ‘me-too’ drugs with limited clinical benefits over existing treatments (which Plazomicin was for UTIs in the U.S.) provide smaller financial returns. As such, Achaogen’s bankruptcy is not necessarily a good example of a broken market for antibiotics, nor should the company’s collapse serve as a justification to pressure governments to establish large-scale pull incentives for the multinational pharmaceutical industry.
The example of Achaogen’s bankruptcy points however to a number of other factors that companies and governments should consider when constructing a viable antibiotics market. These include:
- To get truly novel classes of antibiotics, incentives and subsidies targeted to encourage the development of these need to be a priority.
- Follow-on innovation or repurposing of existing classes of antibiotics that meet a particular urgent need (e.g., overcoming a particular resistance mechanism, or development of a specific formulation, or reducing side effects) can and should also be supported – though on a smaller scale.
- Supporting and investing in innovation in the design of clinical trials and patient recruitment is needed. This includes enlisting more LMICs in trials to generate data for treatment of more complicated resistant infections and data that go beyond the initial market approval requirements, for example, additional evidence to guide the clinical use of the drug.
- Creating a global registration system where countries with the largest health needs are prioritized.
Further references
1, Payne D. Uppsala 2010. *Hit to Phase 2 starts based on GSK data.Phase 2 and Phase 3 success based on Centers for Medicines Research(CMR) 2003 averages for antibacterials (likely based on agents from established classes). #Paul M et al. 2010. Nat Rev Drug Discov 9:203-214.
 This year ReAct is celebrating 15 years of action on antibiotic resistance and this theme article is part of the celebration!
This year ReAct is celebrating 15 years of action on antibiotic resistance and this theme article is part of the celebration!
The story of ReAct started 15 years ago with a small group of people, many who are still with the network today. They all shared a passion for global health, and felt the urgency to address the growing problem of antibiotic resistance. The network has since grown, with the presence of offices in 5 continents and many passionate members working together.
Read more about ReAct 15 years celebrations and learn more about the story of ReAct!
More news and opinion
- Winners ReAct Asia Pacific and Aspic Clubs photo competition 2021
- ReAct Africa Conference: Key takeaways and way forward
- World Health Assembly Special Session 2: Openings for stronger governance of the silent antibiotic resistance pandemic
- Staff interview Juan-Carlos Lopez
- ReAct highlights during World Antibiotic Awareness week 2021
- Staff interview Maria Pränting
- 5 lessons learned from Latin American Summit: Community empowerment – vital for tackling AMR
- The WHA74 Special Session on Pandemic Preparedness and Response – an opportunity to address antibiotic resistance
- ReAct announces the top 15 teams to participate in the online global design sprint Innovate4Health 2021
- City of Hyderabad joins ‘Go Blue’ campaign as part of WAAW Activities
- ReAct Europe and Uppsala University go blue to shed light on the antibiotic resistance issue
- Could the best chemotherapy be an antimicrobial drug?
- Press release: Unique collaboration between Ministry of Health, Zambia and ReAct Africa
- Mobilizing communities to act on antibiotic resistance
- ReAct activities for World Antimicrobial Awareness Week 2021
- Dr Vijay Yeldandi
- 4-day Summit: Latin America discusses the role of the community in National Action Plans on AMR
- The world needs new antibiotics – so why aren’t they developed?
- 3 ways the new WHO costing & budgeting tool supports AMR National Action Plan work
- 5 years after the UN Political Declaration on AMR – where are we now?
- Víctor Orellana
- Local production of vaccines and medicines in focus: Key points from ReAct and South Center UN HLPF side-event
- Behavior change to manage antimicrobial resistance: 8 briefs and 1 webinar-launch by Uppsala Health Summit
- ReAct and ICARS to develop policy guides and tools for low resource settings
- Tapiwa Kujinga, Director of PATAM: In Zimbabwe civil society is involved in every aspect of the response to AMR
- COVID-19: India pays a high price for indiscriminate drug use
- Lancet Global Health article release: Resetting the agenda for antibiotic resistance
- 3 key takeaways for AMR from this year’s World Health Assembly WHA74
- Antibiotic resistance – far more than a medical problem
- UN High-level Dialogue on AMR: political will and investments needed
- Resetting the agenda for antibiotic resistance through a health systems perspective
- 3 questions to newly appointed STAG-AMR members Otridah Kapona and Sujith Chandy
- Walk the talk: time is ticking for all to act on antibiotic resistance!
- Vanessa Carter: 3 years of surviving a drug-resistant infection made me want to create change
- Upcoming ReAct Webinar: Expert Conversation about new report
- ReAct report: Governments need to take more leadership to ensure global sustainable access to effective antibiotics
- 4 considerations for addressing antimicrobial resistance through pandemic preparedness
- Preventing the next pandemic: Addressing antibiotic resistance
- 4 key takeaways from the virtual ReAct Africa Conference 2020
- The threat of the unknown: is lack of global burden data slowing down work on antibiotic resistance?
- ReAct input to the WHO Executive Board Session on Antimicrobial Resistance
- Dr Gautham: informal health providers key to reducing antibiotic use in rural India